Metal sheds and rust information
Do Asgard Sheds rust?
"All this talk of metal sheds and bike storage but doesn't metal just rust?? Anyway, what is rust and how can I stop it?"
One of the most common problems with Metal Outdoor Storage is Rust. A lot of cheap, low-end storage is imported on mass from overseas, and not only is this bad for the UK economy and your carbon footprint, but you will find the finish quality of the metal very low. High security sheds such as the Asgard metal shed range are specially coated to prevent rust from occurring. Asgard Storage is often asked to replace rusty competitors' products after only a year or so of outside use because, with only minimum maintenance, Asgard metal sheds will last decades. For a direct quality comparison - see our best metal sheds page.
Rust Proof Storage Sheds
|
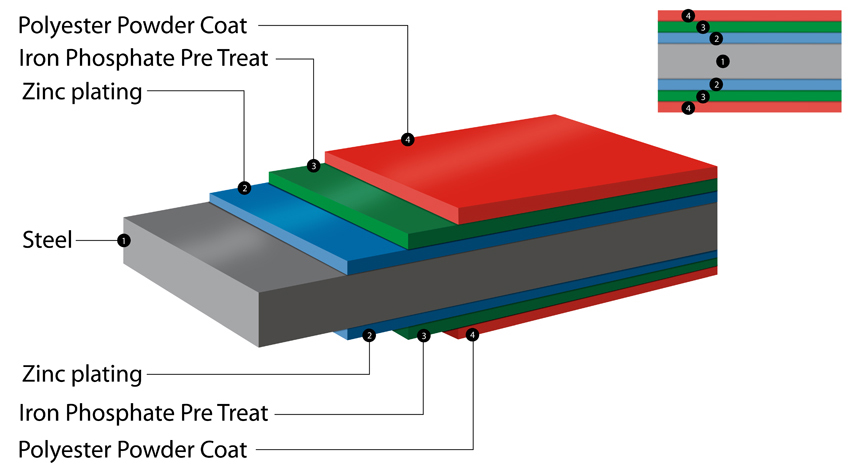
Why are Asgard Sheds Rust Proof? Asgard coat our metal in a polyestor powder coat paint. This makes the steel not only weatherproof but tough and durable. Asgard use only zinc coated screws for shed assembly. |
 |
What is rust and how is it formed?
 |
Now for the technical stuff: |
The main catalyst for the rusting process is dihydrogen oxide, better known as water. Iron or steel structures may appear solid, but water molecules can penetrate the microscopic pits and cracks in any exposed metal. The hydrogen atoms present in water can combine with other elements to form acids, which will eventually cause more metal to be exposed.
If sodium is present, as is the case with saltwater, corrosion will likely occur more quickly. Meanwhile, the oxygen atoms combine with metallic atoms to form the destructive oxide compound. As the atoms combine they weaken the metal, making the structure brittle and crumbly.
|
Some pieces of iron or steel are thick enough to maintain their integrity even if rust forms on the surface. The thinner the metal, the better chance rusting will occur. Water alone does not cause steel to rust, but the acidic reaction allows oxygen to attack vulnerable exposed metal. Placing a steel wool pad in water and exposing it to air will cause almost-immediate rusting; the air around the pad will actually feel several degrees warmer. Eventually the individual iron bonds will be destroyed and the entire pad will disintegrate. Rust formation cannot be stopped easily, but metals can be treated to resist the most damaging effects. Some are protected by water-resistant paints, preventative coatings, or other chemical barriers, such as oil. It is also possible to reduce the chances of rust forming by using a dehumidifier or desiccant to help remove moisture from the air, but this is usually only effective in relatively small areas. |
Avoid sheds that look like this |
Steel is often galvanized to prevent rust from forming; this process usually involves a very thin layer of zinc being applied to the surface. Another process, called plating, can be used to add a layer of zinc, tin, or chrome to the metal. Cathodic protection involves using an electrical charge to suppress or prevent the chemical reaction that causes rust from occurring.
Asgard metal sheds are manufactured from galvanised steel and are designed to last for many decades. All of our sheds come backed with a free 10-year anti-perforation warranty. We've had some fantastic feedback from customers with sheds still going strong after 15 years old. You can see Asgard customer reviews here.
Shop our bike storage sheds, garden sheds or motorcycle garages.
Rust "technical stuff" from wisegeek.com